About Client
A medical device company in Boston, USA specializing in next-generation neuromuscular diagnostics, engaged iOrbit to design and develop a first-of-its-kind clinical-grade integrated EMG-EIM product (iEMG) to improve diagnostics, monitoring, and therapeutic treatment of patients with neuromuscular disorders.
Client’s Challenge
The client had a Proof of Concept (POC) for the iEMG product and was looking to develop a clinical-grade, FDA-ready product with a go-to-market strategy under one roof.


iOrbit Solution
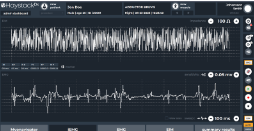
The current product prototype is a Class II clinical diagnostics product that helps both patients and physicians. The Needle iEMG technology provides trackable health data on the muscle’s content and its internal structure.
The iEMG system is operated by a specialized healthcare professional. It records EMG or muscular electric activity, provides information on the internal muscle structure, and helps track disease progression and the therapy effect for drug development.
- 2-channel state-of-the-art design for EMG and impedance measurement
- Simultaneous acquisition of EMG and impedance
- Advanced amplifiers with high-quality data
- Playback function for easy replay of acquired waveforms along with EMG sound
- Optimized communication protocol
As a specification developer, iOrbit conceptualized the iEMG system with the following components:
- iEDX Device connected with a third-party iEMG needle via a cable connector
- iEMG Software, a desktop application running on Windows
- Optional iEMG system data storage on iOrbit cloud platform
iOrbit Scope
- Conceptualized the industrial design for the device and presented three options for review and finalization
- Hardware design, schematic entry, and layout work
- Design decisions regarding processor choices and overall optimization
- Embedded software development in C
- UI/UX design for the desktop application
- Complete design and development of the desktop application
- Creation of DHF as per FDA guidance and iOrbit’s 13485 QMS processes