Case Study
Customer: Leading Medical Device Manufacturer, Netherlands
About Client
A leading medical device manufacturer specializing in pelvic health devices engaged iOrbit to improve the existing clinical professional version of their pelvic health product already in the market.
Client’s Challenge
The existing system was a standalone product with a high Bill of Material. Feedback from the market needed to be incorporated into the application with respect to enhancements and new features for reporting. Being a standalone product, there was no visibility to the client regarding device usage and market reach. The client was unable to monitor the proper usage of probes, which were consumables with limited usage cycles.
iOrbit Solution
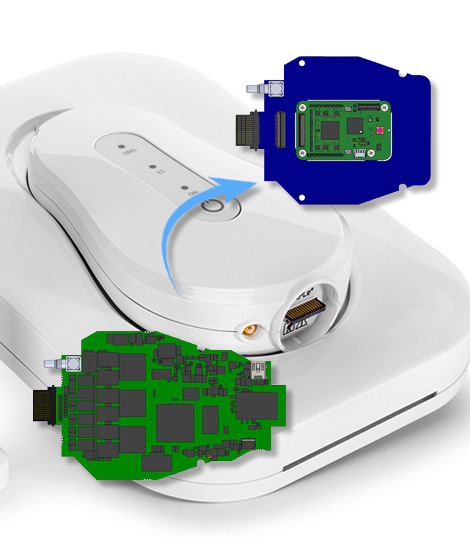
MAPLe Professional Device: The existing system available in the market was a device used for measurement and stimulation of pelvic muscles with the usage of a proprietary probe and a connected application running on an iOS handheld device.
Reduction in BOM: [Add details related to reduction in BOM]
Improvements, Bug Fixes, and Enhancements
The existing CE-certified device and the iOS app running on iOS 12 were in use by clinicians for pelvic floor treatment for their patients. Post-market feedback for the iOS app was collected in the form of bug fixes and improvements to existing functionality. The updated iOS app was expected to be launched in the market in 6 months' time.
iOrbit incorporated the feedback items first-time-right into the iOS app. The app was also made compatible with iOS 13, in addition to iOS 12.
Cloud Connectivity
iOrbit addressed the device usage visibility issues by proposing a connected solution with the integration of iOrbit’s IoMT (Internet of Medical Things) platform. Cloud connectivity helped integrate the clinical version with the at-home use variant for the same set of patients and included probe tracking with a QR code solution as a new feature in the existing iOS application.
Control of Probe Consumables
A proprietary probe was used with the device. The probes were treated as consumables and were expected to be used for a certain number of usage cycles. In the present scenario, it was almost impossible to monitor the usage of these probes at the hands of the clinician, thereby impacting probe sales. iOrbit proposed a QR code-based solution for probe tracking to be used in conjunction with connected cloud.
iOrbit Scope
[Add details related to reduction to BOM activities]
- Designed and developed improvements to the existing iOS app.
- Implemented UI text translations for 6 EU languages.
- Seamless integration of the iOrbit cloud platform with the existing system.
- Designed and implemented connected cloud workflows for providing device usage visibility.
- Designed all workflows ensuring smooth interaction between the clinical version and the at-home variant.
- Designed and implemented the QR code solution for probe usage tracking.
- Thorough regression testing to ensure maintainability of the existing app running on iOS 12.
Key Outcomes
- Reduction in BOM cost by [add value] thereby reducing the cost of manufacturing.
- Cloud integration provided data and analytics to the client that helped in having a bird’s eye view of product usage and improved business decision-making.
- Seamless integration between the clinical and at-home variant immensely benefited both clinicians and patients.
- Having control over probe usage through QR code tracking improved the sale of probes in the existing market.